The Kim Lab
Welcome!
We are looking for motivated Postdocs and Graduate students. Please send your CV to us (junchul.kim@utoronto.ca) if you are interested in joining the lab!

The Kim Lab
Welcome!
We are looking for motivated Postdocs and Graduate students. Please send your CV to us (junchul.kim@utoronto.ca) if you are interested in joining the lab!
A fundamental challenge for both basic and clinical neuroscience is to address how specific neuron types and neural pathways relate to distinct behavioral and physiological responses, therefore, define their function. The main goal of our research program is to address aspects of this question by elucidating the function of specific hippocampal pathways (Part 1) and GABA interneurons subtypes (Part 2) in sensory, emotional and cognitive control in behavioral contexts. Under this overarching theme, we discovered that there are separable circuits and interneuron populations embedded in the hippocampus and prefrontal cortex, working together to guide anxiety, stress, olfaction, and memory, often with opposing functions. In parallel, we have also contributed to the development of new recombinase-based genetic tools that have offered far-reaching capabilities for noninvasively labeling and silencing neuron subtypes of choice.
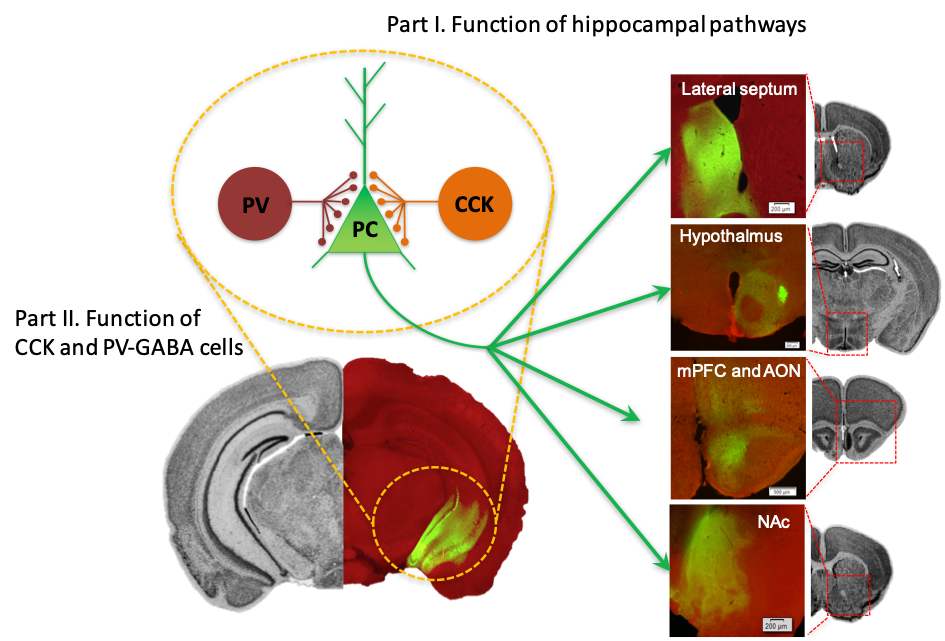
Neural pathways arising from the ventral hippocampus (vHPC). Left, virally-mediated expression of channel rhodopsin-GFP in pyramidal cells (PC) in the vHPC. PV- and CCK-GABA interneurons form synapses on the PC around the soma and proximal dendrites. Right, vHPC-originated axon terminals at its remote targets including the lateral septum, hypothalamus, medial prefrontal cortex (mPFC), anterior olfactory nucleus (AON), and nucleus accumbens (NAc).
The coordinated firing pattern of principal cells, as a neural representation of current and past experiences, is sculpted extensively by local inhibitory interneurons in the brain. The processed neural information then propagates to remote target areas along distinct axon projection pathways to produce diverse behavioural and physiological responses. In part I of our research program, we investigate the role of neural information arising from different hippocampal pathways in behavioural contexts. In part II, we study how hippocampus-dependent behaviours are modulated by the activity of local inhibitory interneurons, focusing on two genetically distinct subtypes of basket cells, namely PV- and CCK-GABA cells.
Part I. Role of hippocampal pathways in shaping anxiety, stress, and olfactory processing.
Much of the study of the mammalian hippocampus has been focused on its role in cognitive functions such as episodic memory and spatial mapping. Emerging research, however, shows that hippocampus is also a major component of circuits underlying emotional control. The dominant view is that these two (cognitive vs. emotional) functions map onto different subregions of hippocampus, where the dorsal part (dHPC) mediates cognitive functions while its ventral portion (vHPC) plays a more influential role in emotional responses. Consistent with the proposed functional heterogeneity within the HPC, the connectivity of the HPC also changes significantly along its dorsoventral axis. The dHPC has strong direct connections to the perirhinal and entorhinal cortex that carry the major inputs of visual and spatial information. On the other hand, the vHPC projects to the prefrontal cortex, hypothalamus, amygdala, nucleus accumbens, as well as other subcortical structures that are associated with fear, anxiety, stress response, and motivation.
Although the accumulated evidence unequivocally shows that the vHPC plays a critical role in controlling emotion, a circuit-level understanding of how it engages in relevant behavioural and physiological responses is lacking. Our working model is that depending on the locus of hippocampal outputs, specific hippocampal pathways subserving different functions are recruited, and each pathway activates a unique set of downstream targets, producing distinct behavioral and physiological outcomes. In this regard, elucidating the function of specific hippocampal pathways will provide an essential entry point for understanding how the hippocampus supports a diverse range of emotional behaviours. Thus, a main objective of my research program (Part 1) is to determine the behavioural function of hippocampal pathways and identify their downstream targets underlying emotional control, with a particular focus on anxiety, stress responses, and olfactory processing.
Part II. Behavioural function of local GABA interneurons
We have shown that HPC outputs to its different remote targets play specialized roles in modulating diverse behavioral processes. Importantly, however, the output signals of the HPC pathways are processed extensively by local GABAergic transmission before they propagate to remote targets. Thus, elucidating the precise function performed by local GABAergic transmission is a critical step in understanding the HPC control of behaviours. The local GABAergic transmission is orchestrated by a range of interneuron subtypes that possess different morphological, electrophysiological and neurochemical properties, and specific connectivity features. Indeed, recent years have witnessed a dramatic accumulation of our knowledge about a division of labor among distinct subtypes of interneuron where distinct interneuron subtypes specialize in inhibiting particular classes of neurons and synapsing on different subcellular compartments at precise time windows.
By far, the most extensively studied interneurons in this regard are the basket cells which synapse on pyramidal cell bodies or proximal dendrites. These basket cells are either parvalbumin (PV)- or cholecystokinin (CCK)-positive, i.e. PV- or CCK-GABA cells. The non-overlapping expression pattern of PV and CCK in basket cells is accompanied by their contrasting firing patterns and synaptic features. PV-GABA cells have fast, non-adaptive firing patterns and form synapses on pyramidal cells predominantly via α1 subunit-containing GABAa receptor with fast IPSC decay kinetics, which makes PV-GABA cells ideal for operating as a non-plastic clockwork in shaping and coordinating fast network oscillation such as gamma rhythm. In contrast, CCK-GABA cells display a slower, more accommodating firing pattern and inhibit pyramidal cells predominantly via α2 subunit-containing GABAa receptor with relatively slow and asynchronous IPSC, which makes them more amenable to synaptic modulation and plasticity and suitable for coordinating slower, theta frequency oscillation.
These contrasting features have stirred speculation that CCK- and PV-GABA neurons serve distinct roles, not only during information processing at a local circuit level but also in shaping task-dependent activity even at behavioural level. Our research program has been tackling this question, using chemo- and optogenetic methods combined with novel recombinase-based gene delivery. Of note, PV-GABA cells have been extensively studied by many labs over the last decade, showing their role in a wide variety of cognitive processes, including gamma rhythm generation, attention, novelty recognition, associative learning and extinction of learned behavior, and working memory, to name a few. In contrast, CCK-GABA cells have been less scrutinized, and their precise role in emotional and cognitive processes remains largely unknown. Addressing this knowledge gap has been a major goal of our research program.

Junchul Kim
junchul.kim@utoronto.ca
2011 - present: Associate Professor, Psychology Department (primary), Cell and Systems Biology Department (cross appointment), University of Toronto
2005~2010 : Postdoctoral Research Fellow, Genetics Department, Harvard Medical School, USA.
2005 : Ph.D. in Molecular biology and Biochemistry, Simon Fraser University, Canada.
1996 : B.Sc. in Biology, Yonsei University, Seoul, Korea.
Teaching: PSY290, PSY390, PSY490

Yoo Kyung (Cindy) Hong
yookyung.hong@mail.utoronto.ca
Dept. of Cell and Systems Biology

Kendall Mar
kendall.mar@mail.utoronto.ca
Dept. of Psychology

Andrew Jiwoo Cheon
andrew.cheon@mail.utoronto.ca
Dept. of Cell and Systems Biology

Ruth Tran
ruth.tran@utoronto.ca
Dept. of Psychology

Stephanie Shishis
stephanie.shishis@mail.utoronto.ca
Dept. of Cell and Systems Biology

Charlotte Romain
charlotte.romain@mail.utoronto.ca
Dept. of Cell and Systems Biology
John Akkus (project student)
john.akkus@mail.utoronto.ca
Jaen Rego (volunteer)
jean.rego@mail.utoronto.ca
Elizabeth Lee Chung (project student)
elizabeth.leechung@mail.utoronto.ca
Fateen Kabir (volunteer)
fateen.kabir@mail.utoronto.ca
Joshua Duong (project student)
joshua.duong@mail.utoronto.ca
Riley Crews (project student)
riley.crews@mail.utoronto.ca
POSTDOCTORAL FELLOWS
Paul Whissell
Gustavo Morrone Parfitt
GRADUATE STUDENTS
Joseph Banning
June Bang
Elly Wong
Victoria Dawson
Afif Aqrabawi
Robin Nguyen
Janine Dominique Cajanding
Sabrina Nawaz
Shadi Bakir
UNDERGRADUATE STUDENTS
Zihe Chen (project student)
Juliet Arsenault (project student)
Chanbee (Emma) So (project student)
Julie Zhao (project student)
Jessica Jenkins (project student)
Aaron Philip Muller (project student)
Ikram Khan (lab manager)
Lena Soukhov (project student)
Golsa Shafa (project student)
Shubham Sharma (project student)
Nicole Fogel (project student)
Chloe Briggs (project student)
Alexander Levit (project student)
Joanna Zhou (project student)
Alex Hsieh (project student)
Nicholas Howell (project student)
Dana Chemali (project student)
Ho-Young Koo (project student)
Eva Qin (project student)
Waldo Lefever (project student)
Wendy Xin (project student)
Cheryl Lau (project student)
Alexander Mulligan (project student)
Jennifer Na (project student)
Laura Park (volunteer)
Inah Park (volunteer)
Dennis Zhang (volunteer)
Katie Dunlop (volunteer)
Grace Cheung (volunteer)
Sandra Vuong (volunteer)
Daniel Jin (volunteer)
Mar KD, So C, Hou Y, Kim JC. Age dependent path integration deficit in 5xFAD mice. Behav Brain Res. 2024 Feb 24;463:114919.
Nguyen R, Sivakumaran Sanghavy, Lambe EK, Kim JC. Ventral hippocampal cholecystokinin interneurons gate contextual reward memory. iScience. 2024 Feb 16; 27(2): 108824.
Bang JY, Sunstrum JK, Garand D, Parfitt GM, Woodin M, Inoue W, Kim JC. Hippocampal-hypothalamic circuit controls context-dependent innate defensive responses. Elife (2022) Apr 14;11:e74736. doi: 10.7554/eLife.74736.
Aqrabawi AJ & Kim JC. Olfactory Memory Representations Are Stored in the Anterior Olfactory Nucleus. Nature Communications. (2020)
Khademullah CS, Aqrabawi AJ, Place KM, Dargaei Z, Liang X, Pressey JC, Bedard S, Yang JW, Garand D, Keramidis I, Gasecka A, Côté D, De Koninck Y, Keith J, Zinman L, Robertson J, Kim JC, Woodin MA. Cortical interneuron-mediated inhibition delays the onset of amyotrophic lateral sclerosis. Brain. 2020 Mar 1;143(3):800-810.
Nguyen R, Venkatesan S, Binko M, Yoon Bang J, Cajanding JD, Briggs C, Sargin D, Imayoshi I, Lambe EK, Kim JC. Cholecystokinin-expressing Interneurons of the Medial Prefrontal Cortex Mediate Working Memory Retrieval. J Neurosci.1919-19. (2020).
Whissell PD, Bang JY, Khan I, Xie YF, Parfitt GM, Grenen M, Plummer NW, Jensen P, Bonin RP, Kim JC. Selective activation of cholecystokinin-expressing γ-aminobutyric acid (CCK-GABA) neurons enhances memory and cognition. eNeuro 12 February 2019, 6 (1).
Aqrabawi AJ & Kim JC. Behavioral evaluation of odor memory in mice. Bio-Protocol. 8, 18 (2018).
Aqrabawi AJ & Kim J. Hippocampal projections to the anterior olfactory nucleus differentially convey spatiotemporal information during episodic odour memory. Nature Communications. 2735 (2018).
Leung C, Cao F, Nguyen R, Joshi K, Aqrabawi AJ, Xia S, Cortez MA, Snead OC 3rd, Kim JC, Jia Z. Activation of Entorhinal Cortical Projections to the Dentate Gyrus Underlies Social Memory Retrieval. Cell Rep. 2018 May 22;23(8):2379-2391.
Dargaeia Z, Bang JY, Mahadevana V, Khademullaha CS, Bedarda S, Parfitt GM, Kim JC, and Woodin MA. Restoring GABAergic inhibition rescues memory deficits in a Huntington's disease mouse model. Proceedings of the National Academy of Sciences 115, E1618–E1626, 2018.
Aqrabawi AJ. & Kim, JC. Topographic Organization of Hippocampal Inputs to the Anterior Olfactory Nucleus. Front. Neuroanat. 12, 12 2018.
Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, Richards BA, Kim JC. Bidirectional Control of Anxiety-Related Behaviours in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology. 2017 Mar 15.
Barrett KT, Dosumu-Johnson RT, Daubenspeck JA, Brust RD, Kreouzis V, Kim JC, Li A, Dymecki SM, Nattie EE. Partial Raphe Dysfunction in Neurotransmission Is Sufficient to Increase Mortality after Anoxic Exposures in Mice at a Critical Period in Postnatal Development. J Neurosci. 2016 Apr 6;36(14):3943-53.
Aqrabawi AJ*, Browne CJ*, Dargaei Z, Garand D, Khademullah CS, Woodin MA, Kim JC. Top-down modulation of olfactory-guided behaviours by the anterior olfactory nucleus pars medialis and ventral hippocampus. *These authors contributed equally to this work. Nature Communications. 2016 Dec 22;7:13721.
Cloke JM, Nguyen R, Chung BY, Wasserman DI, De Lisio S, Kim JC, Bailey CD, Winters BD. A Novel Multisensory Integration Task Reveals Robust Deficits in Rodent Models of Schizophrenia: Converging Evidence for Remediation via Nicotinic Receptor Stimulation of Inhibitory Transmission in the Prefrontal Cortex. J Neurosci. 2016 Dec 14;36(50):12570-12585.
Wasserman DI, Tan JM, Kim JC, Yeomans JS. Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion. Eur J Neurosci. 2016 Jul;44(1):1761-70.
Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, DeBairos D, Kim JC, Cook MN, Dymecki SM. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron. 2015. 88, 774–791.
Britz O, Zhang J, Grossmann KS, Dyck J, Kim JC, Dymecki S, Gosgnach S, Goulding M. (2015). A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. elife (4): e04718.
Whissell, PD, Cajanding, JD, Fogel, N, & Kim JC. Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Front. Neuroanat. 2015 Aug 31;9(124):
Nguyen R, Morrissey MD, Mahadevan V, Cajanding JD, Woodin MA, Yeomans JS, Takehara-Nishiuchi K, Kim JC. Parvalbumin and GAD65 Interneuron Inhibition in the Ventral Hippocampus Induces Distinct Behavioral Deficits Relevant to Schizophrenia. J Neurosci. 2014 Nov 5;34(45):14948-60.
Heimer-McGinn V, Murphy AC, Kim JC, Dymecki SM, Young PW. Decreased dendritic spine density as a consequence of tetanus toxin light chain expression in single neurons in vivo. Neurosci Lett. 2013 Oct 25;555:36-41.
Ray RS*, Corcoran AE*, Brust RD*, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. *Equal contribution. Science. 2011 Jul 29;333(6042):637-42.
Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods In Enzymology. 2010; 477:183-213.
Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 2009; 63 (3):305-15.
Kim JC, Dymecki SM. Genetic fate-mapping approaches: new means to explore the embryonic origins of the cochlear nucleus. Methods Molecular Biology 2009 (493):65-85.
Dymecki SM, Kim JC. Molecular neuroanatomy's "Three Gs": a primer. Neuron 2007; 54 (1): 17-34.
Kim JC*, Badano JL*, Sibold S*, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. *Equal contribution. Nature Genetics 2004 May; 36(5):462-70
Kim JC, Ou Y, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, Leroux MR. (2004) MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. Journal of Cell Science 2005 Mar; 118(5):1007-20.
Ansley JS*, Badano JL*, Blacque OE*, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N (2003). Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. *Equal contribution. Nature 2003 Oct 9; 425(6958): 628-33.
Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Human Molecular Genetics 2003 Jul 15; 12(14): 1651-9.
Han SY, Kim JC, Chung IK Cell type-dependent regulation of human DNA topoisomerase III alpha gene expression by upstream stimulatory factor 2. FEBS Letter 2001 Sep 7; 505(1): 57-62.
Kim JC, Yoon JB, Koo HS, Chung IK (1998) Cloning and characterization of the 5'-flanking region for the human topoisomerase III gene. Journal of Biological Chemistry 273: 26130-7.
Son YS, Suh JM, Ahn SH, Kim JC, Yi JY, Hur KC, Hong WS, Muller MT, Chung IK. Reduced activity of topoisomerase II in an Adriamycin-resistant human stomach-adenocarcinoma cell line. Cancer Chemotherapy and Pharmacology 1998, 41: 353-60.



Lab: (416) 978-3403
Office: (416) 978-4260
Room 4028, Sydney Smith building, 100 St George street
Psychology Department, University of Toronto
Toronto ON, Canada
M5S 3G3
junchul.kim@utoronto.ca